What is a mineral?
To find out whether a mineral is hazardous, we first need to look at what a mineral actually is. A mineral consists of a collection of atoms. Sometimes these are all of one single element, sometimes they are different elements combined together. Atoms are the smallest building blocks of matter that we know. They are also the smallest particles from which a mineral is made. An element is a type of atom with a specific atomic number.
Imagine a tiny little ball. That ball is an atom. An atom is never alone. Everything around us is made up of atoms. An atom will always try to bond with another atom, sometimes with an atom of the same kind, sometimes with one of a different kind. When two or more atoms bond together, we call this a molecule. A molecule can consist of two atoms, but usually it consists of more than two atoms bonded together.
Most minerals are made up of molecules that consist of several different atoms, and therefore of different elements. There are also minerals that consist of bonds between only one type of atom, one element. Think of metals such as gold and silver, but also of sulphur, or of diamond, which is made entirely of carbon.
When you open a book about minerals or look online, you will usually see a chemical formula after the name of the mineral. This formula shows, among other things, which atoms make up the molecule from which the mineral is formed, and how many of each atom are present in one molecule. Each element is abbreviated by one or two letters that are internationally standardised. These abbreviations can be found in the Periodic Table of the Elements, which lists all the elements we know.
Using this, you can determine whether the element in your stone, for example, is a metal. You can also, once you know the name of an element, check whether it poses a health risk, for instance whether it is radioactive.
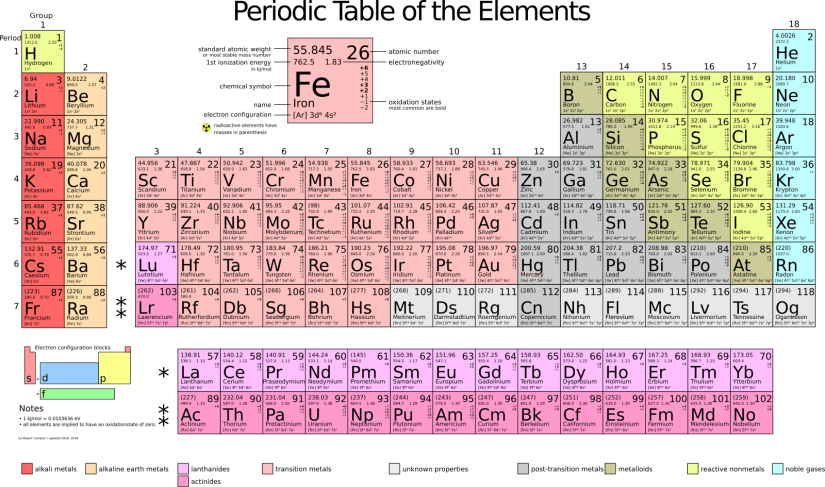
Source Wikimedia Commons
To make it more concrete: fluorite, for example, has the formula CaF₂. This means that two F (fluorine) atoms are bonded to each Ca (calcium) atom. Quartz has the formula SiO₂. In this case, two O (oxygen) atoms are bonded to each Si (silicon) atom. Malachite has the formula Cu₂CO₃(OH)₂. From this you can see that, besides carbon, oxygen and hydrogen, it also contains copper – which is a metal.This means that malachite is not suitable for internal use, and it is therefore better not to put a piece of it in your drinking water.
Besides the elements a mineral is made of, it is also important how these elements are ‘held together’, in other words, what kind of chemical bonds they have. Some bonds are strong, others are very weak. Some minerals are sensitive to dissolving in water or in acids, while others are not at all. There are also substances that only become dangerous when they come into contact with another substance and are then converted into a toxic one (this can also happen inside the body).Substances have three so-called states of matter: gas, liquid and solid. With very few exceptions, in the mineral world we actually only encounter the last one, the solid state. Sometimes a substance can be harmless in one state of matter, while in another state it can be life-threatening.All of these factors influence how toxic a mineral is for us as human beings.
